Gives me a chuckle every time.
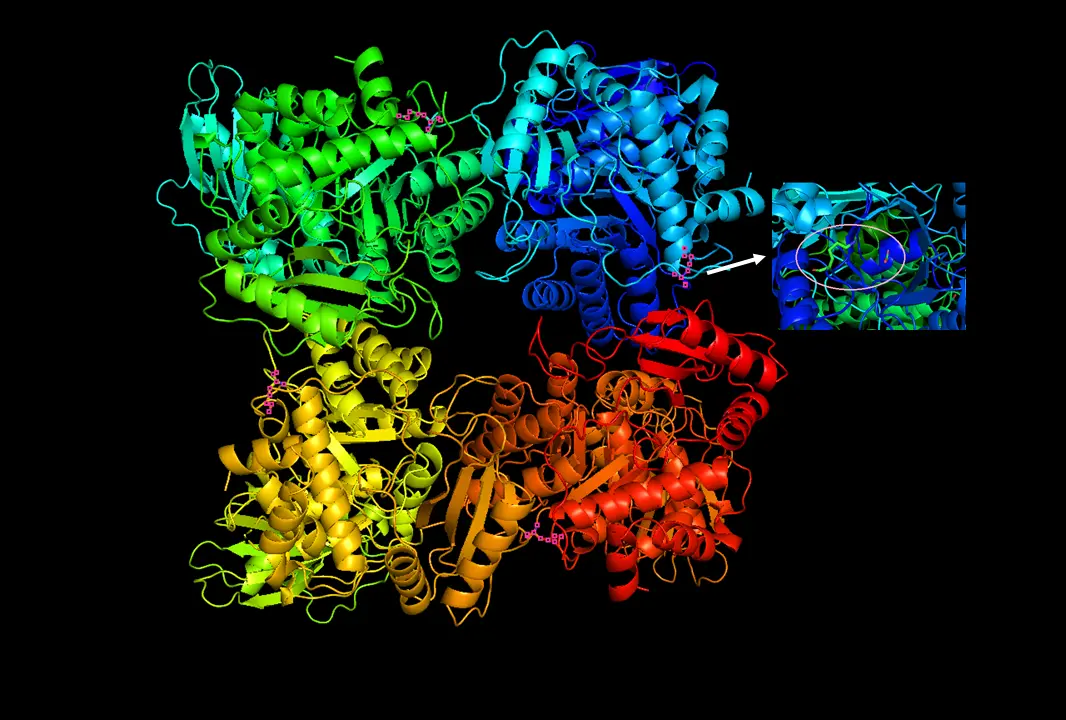
RuBisCO
4 billion years of fixing inorganic carbon in the biosphere. Sometimes mistakes O2 for CO2. Not as fast as some enzymes, but very abundant. Here, have some phosphoglycerates about it.
- 0 Posts
- 17 Comments



Memes don’t have to be funny to be a meme.
Lies, Inc. is another by PKD that will leave your head spinning.
Was this before or after the 3rd season of Westworld?
Love it. One example that springs to mind is calling out the NCAA.
“‘Student Ath-uh-letes’? Haha, that is brilliant, sir.”
 6·8 months ago
6·8 months ago- Easy access to dark skies for stargazing.

 8·8 months ago
8·8 months agoPesticides

 2·9 months ago
2·9 months agoI could also be wrong, but I believe SDS has less ‘affinity’ for protons than acetic acid (which is part of the reason why detergents work so well). You’d need sulfuric acid, or something stronger, and removal from solution of its buddy ion sodium. Then I think you could protonate dodecyl sulfate.
Now acetic acid and soaps…yeah, far more likely to generate scum. The polar head is a weaker acid.
The importance of soap to human civilization is documented by history, but some problems associated with its use have been recognized. One of these is caused by the weak acidity (pKa ca. 4.9) of the fatty acids. Solutions of alkali metal soaps are slightly alkaline (pH 8 to 9) due to hydrolysis. If the pH of a soap solution is lowered by acidic contaminants, insoluble fatty acids precipitate and form a scum. A second problem is caused by the presence of calcium and magnesium salts in the water supply (hard water). These divalent cations cause aggregation of the micelles, which then deposit as a dirty scum.
These problems have been alleviated by the development of synthetic amphiphiles called detergents (or syndets). By using a much stronger acid for the polar head group, water solutions of the amphiphile are less sensitive to pH changes. Also the sulfonate functions used for virtually all anionic detergents confer greater solubility on micelles incorporating the alkaline earth cations found in hard water.

 15·11 months ago
15·11 months agoWhen things get really tough, two of you will double up on the same keyboard.
1 in 6 have multiple personalities and substance abuse daemons.
Your bosses ride little skateboards everywhere, when they’re not busy programming animated singing viruses.
The FBI watches you code, but has no idea what they’re looking at.
A significant fraction of you can type with your feet, proficiently.




https://en.wikipedia.org/wiki/Meme